This guidance document is part 1 of our series “Protection without pollution: COVID-19 waste-reduction strategies.” These three guides will help health systems, hospitals, and other health care providers set up successful immunization programs that protect the health of people and our planet.
Gloves are the highest-volume disposable product purchased by health care. Glove use has increased dramatically and is expected to nearly double in the next five years. As health care facilities rethink their purchasing and supply chains in the wake of COVID-19, this guidance can serve as a roadmap for advancing sustainable options in the marketplace.
Our recommendations
|
Target criteria
Health Care Without Harm presents the sustainable glove criteria to support supply chain professionals with sustainable purchasing and help health care institutions meet their goals. Currently, most purchasing requirements for gloves do not include broad sustainability criteria.
Together, hospitals around the globe can use their purchasing power to increase demand for products that reduce harm for people and the planet. The criteria includes examination and surgical gloves.
Glove purchasing considerations
How to establish your purchasing priorities
Develop a contract clause with your supplier that sets goals and timelines, and requires reports on progress toward the achievement of desired environmental and social criteria. Ask your supplier for the Greenhealth Approved seal. Examination and surgical gloves that carry the seal have been vetted and found to meet the Sustainable Gloves criteria.
- Reduce unnecessary waste of gloves through packaging improvement (i.e. when taking a glove out of the package, others should not fall out) A study in Sweden showed that 6% of gloves were lost due to poor packaging, increasing costs, and waste.
- The supplier/bidder should report the results of completed Code of Conduct audits of factories that manufacture gloves. The audit should be no more than 2-years old and be performed according to established methods such as SA8000, SMETA IV pillar, BSCI, etc.
- The purchaser/tenderer should report which risks have been identified in the audit and how these risks have been assessed in the supply chain for offered gloves
- At the start of the contract, the manufacturer should specify the constituent substances that have either been added during manufacture or are already known to be included in the product; as accelerators or antioxidants that are known to cause health effects based on available data (see "Chemicals and allergens in the manufacture of disposable gloves").
- The product should not include chemicals that have a harmonized classification as skin sensitizers under the Classification, Labelling and Packaging (CLP) Regulation such as chromium VI, nickel, and cobalt compounds. See ECHA announcement and Annex XV proposing restrictions on skin sensitizing substances, Table 19 pages 108-128)
- The weight of gloves should be standardized and disclosed. Products with the lowest unit weight value should be preferred while meeting quality standards
- Information should be provided on the availability of environmental management systems (its scope should include the manufacturing process of the product), for example, ISO 14001.
- Documentation should be provided regarding greenhouse gas emissions (carbon footprint must include scope 1, 2, and 3 emissions and third-party verification). The bidder should specify the methods used (scope includes the manufacturing process of the product), for example, disclosure through the Carbon Disclosure Project (CDP) or others using the Greenhouse Gas Protocol.
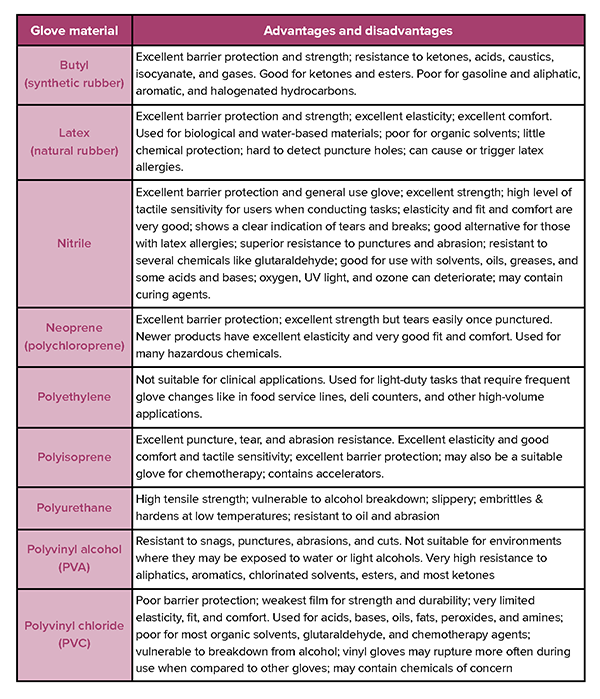
Materials
Product purchasing and databases
Products that carry the seal have been through a review process and have been found to meet the criteria established by Health Care Without Harm and Practice Greenhealth for sustainability.
Additionally, the following product databases can serve as good starting points to find sustainable gloves, but do not necessarily reflect gloves that meet these criteria.
- Health Care Without Harm Europe’s Safer Medical Devices Database covers a range of products and lists alternatives that do not contain PVC, phthalates, and BPA.
- U.S General Services Administration’s Sustainable Facilities Tool database contains lists of gloves that do not contain PVC, DEHP, other phthalates, and latex.
Considerations
Usage
- Hand hygiene is the most critical intervention to protect against pathogens and health care-acquired infections
- Choose gloves that are appropriate for their intended use. Glove selection and usage guidance provide more information on how to select the right glove for the task. For example, the barrier protection required of biologics, radioactive material, or chemicals must be matched with the appropriate glove material.
- Gloves are only one component of hand hygiene. They should be used only where they have been demonstrated to reduce contamination for either the practitioner or the patient.
- Gloves should not be used for routine duties. For example, you do not need to wear gloves to administer solid medication. Instead, practitioners should use the aseptic non–touch technique.
- Gloves should be removed immediately after a procedure to prevent cross-contamination. Hands should then be decontaminated.
- Evidence suggests that gloves can be used inappropriately in clinical practice. Improper use of non-sterile gloves can lead to cross-contamination and has been implicated in infection outbreaks. Gloves are often used when they aren’t needed, put on too early, taken off too late, or not changed at critical points.
- Research shows that patients often feel uncomfortable with inappropriate use of gloves for personal tasks.
Sterile vs. non-sterile glovesSterile and non-sterile (exam) gloves each have a distinct purpose. Sterile gloves are used to protect the patient from the practitioner Non-sterile gloves are used to protect the patient, practitioner, or other users, when there is direct contact with hazardous chemicals, body fluids, non-intact skin, or where contact with mucous membranes is anticipated. |
Occupational health and allergy concerns
- Half of all health care workers may experience dermatitis in any year. Approximately 1 in 5 nurses develop hand dermatitis – a painful, debilitating condition that may require staff to be moved out of clinical areas.
- Allergenic ingredients in gloves can cause Type 1 and Type 3 hypersensitivity reactions, depending on the agent. It is important to diagnose the allergic reaction correctly to choose the appropriate gloves for the practitioner
- For latex gloves: closely monitor allergy concerns including information on protein content in latex gloves and the extent of powdered content in all gloves.
- Some practitioners may be allergic to the accelerants used in many gloves.
- Some components of gloves can pose a threat to patients and workers.
- Ortho-phthalates are added to PVC and other plastics to impart flexibility. They are used in many products so exposure is widespread, and can be cumulative. Adverse effects include hormone disruption, reproductive and developmental impacts, and kidney toxicity. Exposure to some ortho-phthalates is associated with an increased risk of asthma.
- Some biocides used in gloves can be dangerous or toxic to humans and the environment and can accelerate the development of resistance to bacteria.
- Many gloves are made with accelerants like thiurams, thiazoles, and carbamates that are contact allergens and can cause skin irritation and/or sensitization.
Environment and health
- Reducing glove use where possible eliminates the resources and waste associated with unnecessary use.
- Gloves are the highest volume disposable product purchased by health care. Its use has increased dramatically and is expected to nearly double in the next five years.
- Manufacture and transport of gloves require resources and energy and require the use of chemicals of concern.
- Glove disposal results in waste that, if handled improperly, can threaten health.
- A pilot project in the United Kingdom’s National Health Service showed glove use could be dramatically reduced with significant savings and carbon reduction while maintaining infection prevention and improving care.
- Some materials used to manufacture gloves can be toxic throughout their life cycle
- PVC is toxic throughout its life cycle. It is derived from vinyl chloride, a known human carcinogen. Every step in the production of PVC involves the use of chemicals of high concern. Burning PVC gloves can result in the formation of highly toxic chemicals.
- The manufacture and disposal of gloves can threaten surrounding communities and workers.
- Recycling PVC is challenging and can hinder the recycling of other kinds of plastic.
Ethical
Recent reports have documented worker exploitation around glove manufacture including forced labor, poor working conditions, and debt bondage. The U.S. Customs and Border Protection agency barred some products from being distributed in the country after finding "reasonable evidence" that the companies were using forced labor. Allegations of abuse in glove production also include passport confiscations, illegal withholding of pay, and restricted freedom of movement.
As a result, it is important to:
- Research glove sourcing
- Require suppliers to have effective risk management regarding workers' rights in accordance with the ILO's core conventions in their operations and in the supply chain of subcontractors who directly participate in fulfilling the contract
Innovation needed
This is an active area of research. Researchers, suppliers, manufacturers, and purchasers should continue to learn more and better understand the specific chemicals being used in gloves and to strive to find and bring into production safer alternatives.
- Develop new non-fossil fuel-based materials
- Eliminate accelerants in the final products.
- Optimize manufacturing to reduce material used (for example reduce weight and thickness) while maintaining high-performance standards.
- Create circular systems to recover and recycle gloves and to manufacture gloves that are easily recyclable (observe circular economy and extended producer responsibility principles).
- Create high-performing gloves that can be reused.
- Life cycle methodologies and quality differ from manufacturer to manufacturer. Innovation is needed to improve, standardize and strengthen life-cycle analyses.
Learn more
- Health Care Without Harm: “Polyvinyl chloride in health care: A rationale for choosing alternatives”
- Health Care Without Harm Europe: Safer procurement resources
- Health Care Without Harm Europe: Non-Toxic Health Care: Alternatives to Hazardous Chemicals in Medical Devices: Phthalates and Bisphenol A
- Health Care Without Harm Latin America: Chemical substances resources
- CDC: Preventing Allergic Reactions to Natural Latex Rubber in the Workplace
- CDC: Latex Allergy Prevention Guide
Case studies
- “Gloves are off” campaign case study, Great Ormond Street Hospital, National Health Service, England
- “Preventing Harm from Phthalates, Avoiding PVC in Hospitals”
- The Vienna Hospital Association and Stockholm County Council, page 16
- Na Homolce Hospital Czech Republic, page 17
- Kaiser Permanente moves away from PVC gloves
- “Reducing The Carbon Footprint of Healthcare Through Sustainable Procurement”
- Single-use health care products in Sweden, page 8
- Single-use health care products in Sweden, page 8
